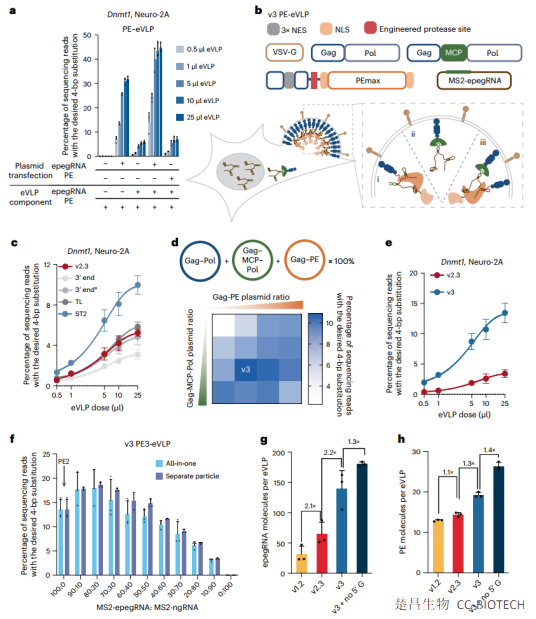
Pilot editing can precisely install genomic replacements, insertions and deletions in living systems. However, efficient delivery of lead editing components in vitro and in vivo remains a challenge.
On January 8, 2024, the team of David R. Liu from the Broad Institute published a research paper titled "Engineered virus-like particles for transient delivery of prime editor ribonucleoprotein complexes in vivo" online in Nature Biotechnology. The research report developed lead editor engineered virus-like particles (PE-eVLPs), which can deliver lead editing proteins, lead editing guide RNAs, and single guide RNAs as transient ribonucleoprotein complexes.
Compared with PE-eVLP structures based on previously reported base editor eVLP architectures, we systematically designed v3 and v3b PE-eVLPs with 65- to 170-fold improvement in editing efficiency in human cells. In two mouse models of genetic blindness, a single injection of v3b PE-eVLPs resulted in treatment-related levels of lead editing in the retina, restoration of protein expression and partial recovery of visual function. Optimized PE-eVLPs support transient delivery of lead editing ribonucleoproteins in vivo, improving the potential safety of lead editing by reducing off-target editing and eliminating the possibility of oncogenic transgene integration.